
Our lead candidate AN2025, is a pan-PI3K inhibitor currently undergoing a global Phase III trial. In-licensed from Novartis, we have the exclusive global rights to develop and commercialize AN2025. It is currently the most advanced drug candidate in registrational clinical trial phase for the treatment of recurrent or metastatic HNSCC after disease progression with anti-PD-1/PD-L1 therapy. We believe that AN2025, if approved, has the potential to be the first global product with a label to address this unmet medical need and capture a sizable addressable market. In April 2023, the Company entered into an option agreement with Nippon Kayaku to further advance the commercialization of this candidate in Japan.
AN2025 BURAN Phase 3 Trial in r/m HNSCC After Anti-PD-1/PD-L1 treatment
(NCT04338399)
The BURAN study is a randomized, open-label Phase 3 study assessing the treatment effect of once-daily AN2025 in combination with weekly paclitaxel compared to weekly paclitaxel alone in patients with r/m HNSCC that have progressed after:
Prior anti-PD-L1 monotherapy;
Prior anti-PD-L1 therapy in combination with platinum-based therapy; or after
Sequential treatment of anti-PD-L1 therapy, either prior to or post platinum-based therapy
In November 2023, we announced the completion of patient enrollment in the phase III clinical trial at more than 180 sites around the world, spanning 18 markets in North America, Europe, Asia, and South America. Enrolled patients are randomized in a 2:1 ratio to receive either daily AN2025 (100 mg) in combination with weekly paclitaxel (80 mg/m2) or weekly paclitaxel alone in a 21-day treatment cycle. The primary endpoint of this study is OS for the entire (intent-to-treat) population of patients. Secondary endpoints include safety profile and other efficacy measurements such as PFS, ORR, DoR, and HRQoL.
BERIL-1 Phase 2 Study for r/m HNSCC(NCT01852292)1,2
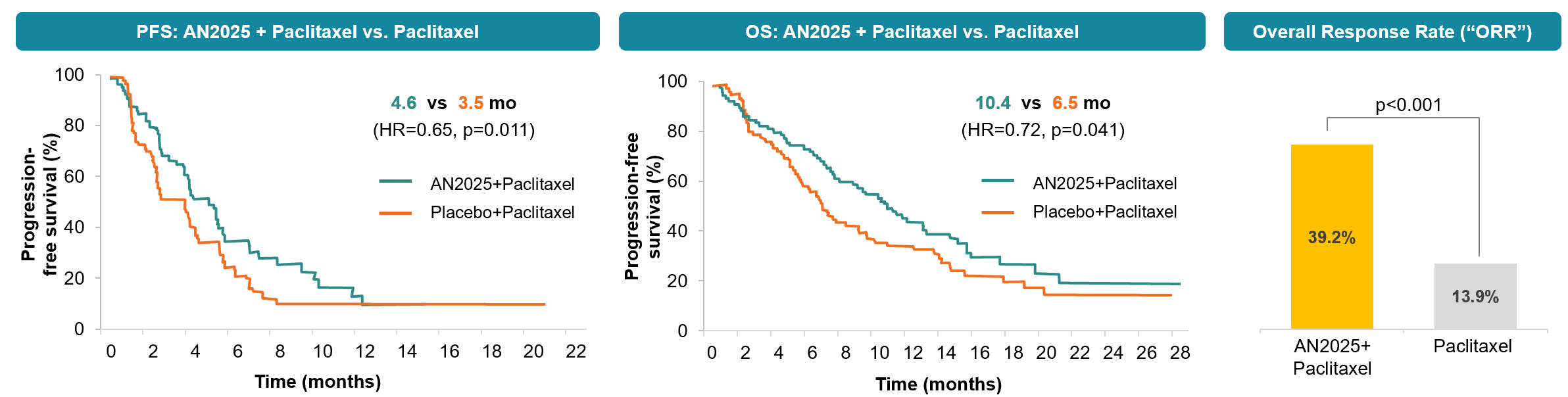
The trial was a multi-center, randomized, double-blind, placebo-controlled Phase II study assessing patients with histologically or cytologically-confirmed recurrent or metastatic HNSCC after disease progression on or after one previous platinum-based chemotherapy regimen in the metastatic setting. A total of 158 eligible patients were enrolled from 58 centers across 18 jurisdictions and randomly assigned (1:1) to receive second-line oral AN2025 (n=79, 76 treated with 100 mg once daily) or placebo (n=79, 78 treated with 80 mg/m2 on days 1, 8, 15 and 22) plus intravenous paclitaxel in 28-day treatment cycles.
The clinical data showed that the combination of AN2025 with paclitaxel achieved an mOS of over 10 months (vs. 6.5 months in the placebo plus paclitaxel group), an mPFS of 4.6 months (vs. 3.5 months in the placebo plus paclitaxel group), and a 39.2%ORR (vs. 13.9% in the placebo plus paclitaxel group).
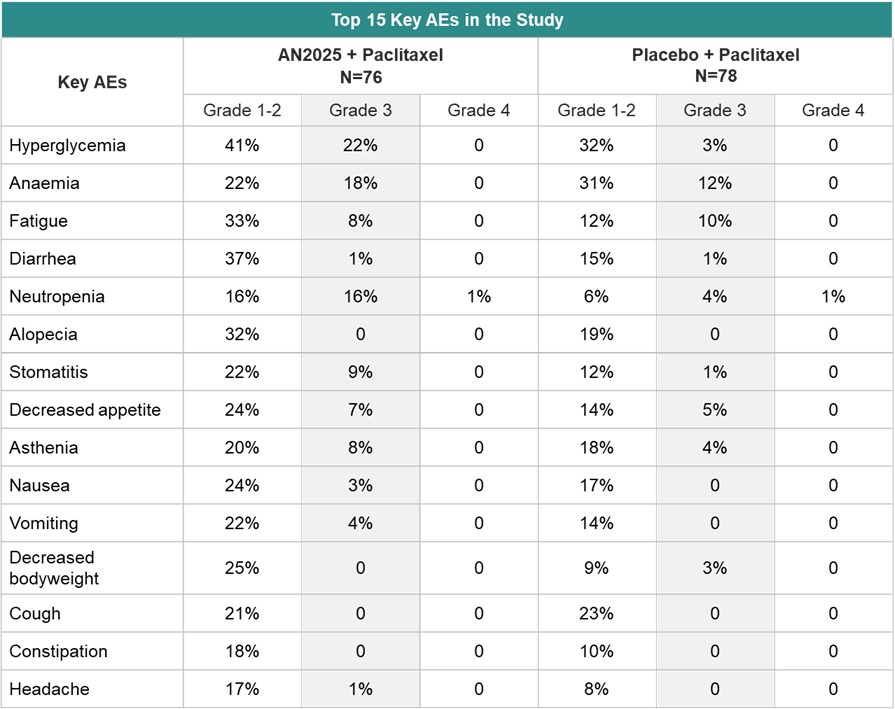
The safety data demonstrated that when AN2025 was combined with paclitaxel, grade 3-4 adverse events (“AEs”) (82% in the AN2025 plus paclitaxel group vs. 72% in the placebo group), serious adverse events (“SAEs”) (57% in the AN2025 plus paclitaxel group vs. 47% in the placebo group) or on-treatment deaths (20% in the AN2025 plus paclitaxel group vs. 22% in the placebo group) is comparable to paclitaxel alone. The most common grade 3–4 adverse events were hyperglycaemia (17 [22%] of 76 patients in the buparlisib group vs two [3%] of 78 patients in the placebo group), anaemia (14 [18%] vs nine [12%]), neutropenia (13 [17%] vs four [5%]), and fatigue (six [8%] vs eight [10%]).
Based on the clinical results, we received Fast Track designation from the FDA for the investigation of AN2025 in recurrent or metastatic HNSCC with disease progression on or after platinum-based therapy.
Reference
1.Lancet Oncol,2017,323-335(pdf).
2.Clin Cancer Res,2018,2025-2516(pdf).