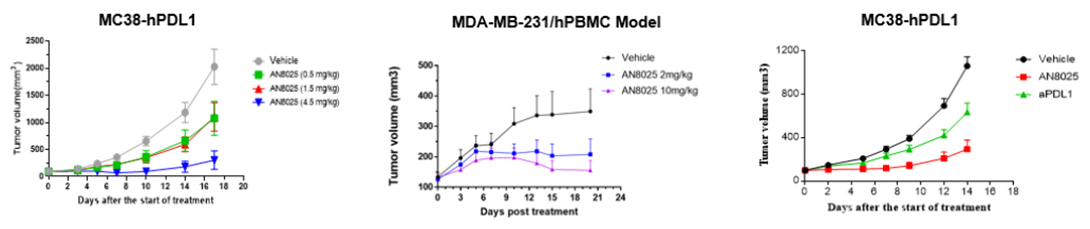
AN8025 emerges as a tri-specific antibody fusion protein derived from a clinically validated and approved αPD-L1 antibody, fused with functionally optimized CD86 variant (CD86v) and LAG3 variant (LAG3v) on its C- and N-terminus of heavy chain, respectively. AN8025 leverages the LAG3 component to stimulate the differentiation and maturation of antigen-presenting cells (APCs), while the CD86 and αPD-L1 components synergistically amplify tumor-specific T cell responses. Together, these actions orchestrate a multifaceted enhancement of immune function. The clinical phase I study of AN8025 is currently ongoing.
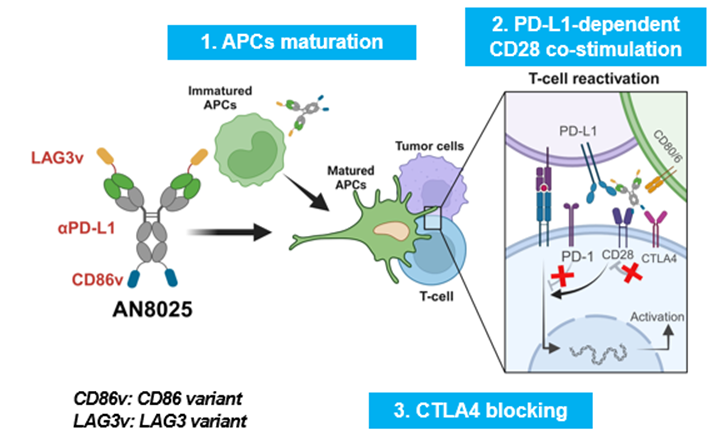
In vitro, we demonstrated that AN8025 improved the quantity and quality of APCs, compared to an aPD-L1 mAb.
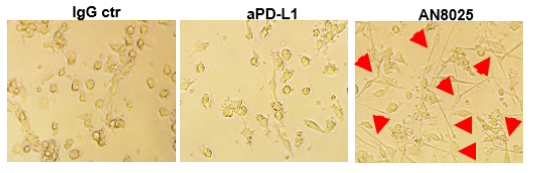
We also demonstrated that AN8025 induced stronger T Cell response than an aPD-L1 mAb and displays PD-L1-dependent T-cell activation. Additionally, this elevated T cell activation is not observed in human primary T cells, where PD-L1 levels are relatively low, unlike the unconditional activation of T cells by the CD28 super-agonist.
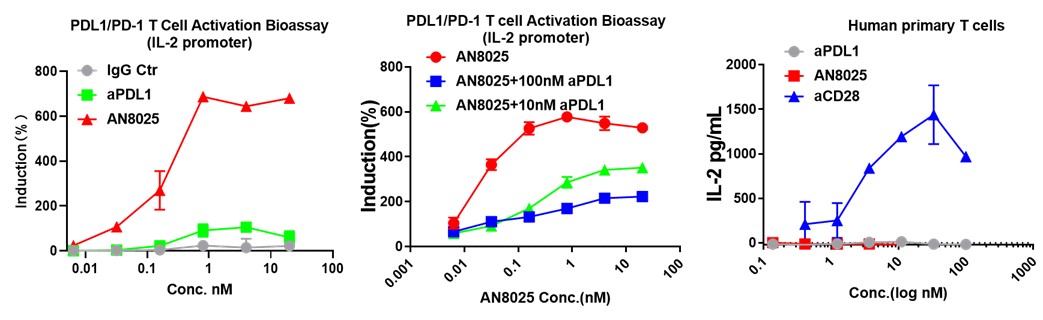
In addition, we demonstrated that AN8025 displayed potent antitumor efficacy in syngeneic and humanized mouse tumor models.